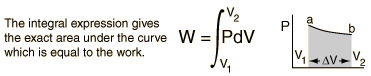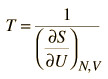| Thermo Chemistry / Thermo Dynamics | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
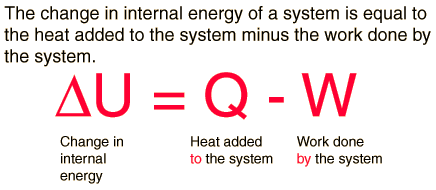
First Law of ThermodynamicsThe first law of thermodynamics is the application of the conservation of energy principle to heat and thermodynamic processes:
The first law makes use of the key concepts of internal energy, heat, and system work. It is used extensively in the discussion of heat engines. It is typical for chemistry texts to write the first law as ΔU=Q+W. It is the same law, of course - the thermodynamic expression of the conservation of energy principle. It is just that W is defined as the work done on the system instead of work done by the system. In the context of physics, the common scenario is one of adding heat to a volume of gas and using the expansion of that gas to do work, as in the pushing down of a piston in an internal combustion engine. In the context of chemical reactions and process, it may be more common to deal with situations where work is done on the system rather than by it. EnthalpyFour quantities called "thermodynamic potentials" are useful in the chemical thermodynamics of reactions and non-cyclic processes. They are internal energy, the enthalpy, the Helmholtz free energy and the Gibbs free energy. Enthalpy is defined by H = U + PV where P and V are the pressure and volume, and U is internal energy. Enthalpy is then a precisely measurable state variable, since it is defined in terms of three other precisely definable state variables. It is somewhat parallel to the first law of thermodynamics for a constant pressure system Q = ΔU + PΔV since in this case Q=ΔH
The internal energy U might be thought of as the energy required to create a system in the absence of changes in temperature or volume. But if the process changes the volume, as in a chemical reaction which produces a gaseous product, then work must be done to produce the change in volume. For a constant pressure process the work you must do to produce a volume change ΔV is PΔV. Then the term PV can be interpreted as the work you must do to "create room" for the system if you presume it started at zero volume.
System WorkWhen work is done by a thermodynamic system, it is usually a gas that is doing the work. The work done by a gas at constant pressure is:
For non-constant pressure, the work can be visualized as the area under the pressure-volume curve which represents the process taking place. The more general expression for work done is:
Work done by a system decreases the internal energy of the system, as indicated in the First Law of Thermodynamics. System work is a major focus in the discussion of heat engines.
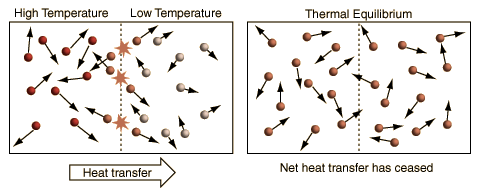
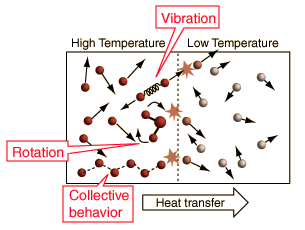
A More General View of TemperatureWhen a high temperature object is placed in contact with a low temperature object, then energy will flow from the high temperature object to the lower temperature object, and they will approach an equilibrium temperature. When the details of this common-sense scenario are examined, it becomes evident that the simple view of temperature embodied in the commonly used kinetic temperature approach has some significant problems.
The above illustration summarizes the situation when the kinetic temperature gives a reasonable general description of the nature of temperature. For monoatomic gases acting like point masses, a higher temperature simply implies higher average kinetic energy. Faster molecules striking slower ones at the boundary in elastic collisions will increase the velocity of the slower ones and decrease the velocity of the faster ones, transferring energy from the higher temperature to the lower temperature region. With time, the molecules in the two regions approach the same average kinetic energy (same temperature) and in this condition of thermal equilibrium there is no longer any net transfer of energy from one object to the other.
Complications such as these have led to the adoption of a different approach to the concept of temperature in the study of thermodynamics. Schroeder's proposal for a theoretical definition of temperature is:
The kinetic temperature for monoatomic ideal gases described above is consistent with this definition of temperature for the simple systems to which it applies. In that case the equilibrium reached is one of maximum entropy, and the rate of approach to that state will be proportional to the difference in temperature between the two parts of the system. Noting that the equilibrium state of a collection of particles will be the state of greatest multiplicity, then one can define the temperature in terms of that multiplicity (entropy) as follows:
Temperature is expressed as the inverse of the rate of change of entropy with internal energy, with volume V and number of particles N held constant. This is certainly not as intuitive as molecular kinetic energy, but in thermodynamic applications it is more reliable and more general.
Specific Heat Capacities Table
Values are at Room Temperature and Atmospheric Pressure unless otherwise stated.
SPECIFIC HEAT HOMEWORK PROBLEMS: 1) When a 50.0 gram piece of nickel absorbs 350.0 J of heat, the temperature of the nickel changes from 20.0 0C to 36.0 0C. What is the specific heat of nickel? 2) A solar-heating specialist is considering paraffin as a possible solar-heat collector. How many kilograms of paraffin would be needed to collect as much energy as 4.78 x 103 kg of water? The specific heat of paraffin is 2.9 J / g 0C. (Hint: Assume the same temperature change.) 3) Calculate the quantity of heat that must be removed from 200.0 grams of ethyl alcohol, C2H6O to cool it from 25.0 0C to 10.0 0C. (ethyl alcohol = 2.45 J / g 0C) 4) How much energy is required to heat a #10 iron nail with a mass of 7.0 grams from 25.0 0C until it becomes red hot at 750 0C? 5) If 5750 J of energy are added to a 455 gram piece of granite at 24.0 0C, what is the final temperature of the granite? 6) A 30.0 gram sample of an unknown metal is heated from 22.0 0C to 59.2 0C. During the process, 1.00 kJ of energy is absorbed by the metal. What is the specific heat of the metal? SPECIFIC HEAT PRACTICE ANSWERS: 1) 0.438 J / g 0C 2) 6.9 x 103 kg paraffin 3) 7350 J or 7.35 kJ 4) 2.3 kJ 5) 39.7 0C 6) 0.896 J / g 0C
E-mail the Answers or bring it to the next class.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||